Regs Coming Through the End of the Year: Expect the Expected and the Unexpected
McDermott+ is pleased to bring you Regs & Eggs, a weekly Regulatory Affairs blog by Jeffrey Davis. Click here to subscribe to future blog posts.
October 17, 2024 – There could be an all-you-can-eat buffet of regs (and eggs) from now until the end of the year!
As you may recall, a July 2024 Regs & Eggs blog post highlighted the spring 2024 “unified agenda” that listed all the regs that the Biden Administration planned to issue in 2024 and beyond. Since then, some of the regs in the agenda have been published, and others seem likely to be released on schedule towards the end of the year (we have no reason to think otherwise). However, several regs have been added to the queue that weren’t on the agenda at all. These regulatory “surprises” always keep us in the health policy world on our toes!
So, let’s take stock of some of the big healthcare regs (both the expected and the unexpected) that may come out from now through the end of the year.
Expect the Expected
- Calendar Year Payment Regs: All of the calendar year 2025 Medicare payment regs are expected to be released on or around November 1, 2024, with policies and payment rates becoming effective on January 1, 2025. These include the following:
- Physician Fee Schedule.
- Hospital Outpatient Prospective Payment System.
- Home Health Prospective Payment System.
- End-Stage Renal Disease Prospective Payment System.
- Increasing Organ Transplant Access (IOTA) Model Final Reg: The IOTA model, proposed in May 2024, is a mandatory Medicare payment model that aims to test whether performance-based incentive payments paid to or owed by participating kidney transplant hospitals increase patient access to kidney transplants. The White House Office of Management and Budget (OMB) is currently reviewing the final reg that would implement the model. OMB review is the last stage of the federal government’s “clearance” process before a reg is released. Regs in this stage can be in OMB clearance for days, weeks, or months. While we don’t know how long the reg will be under review, the Centers for Medicare & Medicaid Services (CMS) initially proposed a model start date of January 1, 2025 – so if that is going to stick, the reg may be issued pretty soon!On a related note, CMS is also expected to release a proposed reg in the coming months with clarifications and technical modifications to the standards used to evaluate and recertify organ procurement organizations and to the competition and decertification process for organ procurement organizations.
- Coverage of Certain Preventive Services Under the Affordable Care Act (ACA) Final Reg: This is another reg currently in OMB clearance that could be released shortly. The reg would finalize proposed changes to religious and moral exemptions and accommodations regarding coverage of certain preventive services under the ACA. The unified agenda listed the expected release date as November 2024.
- No Surprises Act Independent Dispute Resolution (IDR) Operations Final Reg: The IDR operations final reg makes important changes intended to improve the No Surprises Act federal IDR process. Stakeholders initially anticipated that the final reg would be released this summer, with policies becoming effective at the end of 2024 or in early 2025. However, to the frustration of many stakeholders, the unified agenda listed the final reg’s expected release date as November 2024. The reg is not yet at OMB for final review, so it’s hard to know whether it will actually be released in November or be delayed further.
- Establishment of New Safe Harbors Under the Federal Anti-Kickback Statute Proposed Reg: CMS is expected to release a proposed reg by November that would create:
- A safe harbor to protect certain mental health and behavioral health improvement or maintenance programs provided by hospitals, ambulatory surgical centers, community health centers, rural emergency hospitals, and skilled nursing facilities.
- Another safe harbor that would protect certain evidence-based contingency management programs for the treatment of substance use disorders.
- Interoperability Standards and Prior Authorization for Drugs Proposed Reg: In November CMS is also expected to release a proposed reg that would create new requirements for Medicare Advantage (MA) organizations and qualified health plans offered on the federally facilitated exchanges to streamline processes for prior authorization of certain drugs. CMS stated in the unified agenda that it is developing this reg in part because of the many public commenters who responded to the CMS Interoperability and Prior Authorization Reg and urged the agency to expand the prior authorization policies to include drugs. This reg is not yet at OMB for review.
- Modifications to the HIPAA Security Rule to Strengthen the Cybersecurity of Electronic Protected Health Information Proposed Reg: CMS could release a proposed reg by the end of the year that aims to improve cybersecurity in the healthcare sector by strengthening requirements for Health Insurance Portability and Accountability Act (HIPAA) regulated entities to safeguard electronic protected health information and prevent, detect, contain, mitigate, and recover from cybersecurity threats. While the proposed rule was originally slated to come out this year, it is unclear whether it might be pushed to 2025.
- Health Data, Technology, and Interoperability: Patient Engagement, Information Sharing, and Public Health Interoperability (HTI-2) final reg: In July 2024, the Office of the National Coordinator for Health Information Technology, now known as the Assistant Secretary for Technology Policy (ASTP), published a proposed reg that would update criteria for certified health information technology and revise certain information blocking requirements. While the public comment period closed just this month, ASTP could finalize the reg by the end of 2024 or in early 2025. Many stakeholders commented that the timelines in the proposed reg were overly ambitious, which may impact the final reg’s ultimate publication date.
Expect the Expected (Sort of)
This is an election year (in case you needed a reminder), so it is the Biden Administration’s last year to put its mark on some important healthcare programs. And because this is the administration’s last chance to issue regs, it may, like prior administrations, speed up its usual rulemaking process in certain cases. For example, while CMS signaled in the unified agenda that it would release regs affecting the ACA marketplaces (both state marketplaces and the federally facilitated exchanges) and MA and Medicare Part D plans for 2026 (thus the regs are “expected”), it is unusual for CMS to establish policies for these programs so early – except during an election year. Just as the Trump Administration established final MA and Part D policies for 2022 on January 15, 2021, five days before the change in administration, the Biden Administration could follow suit.
CMS just released the 2026 ACA proposed notice of benefit and payment parameters two weeks ago. This reg notably includes enforcement-related proposals that aim to crack down on agent and broker practices that CMS believes potentially harm or confuse consumers (these types of “program integrity” and consumer protection proposals have been a priority for this administration). CMS could release 2026 ACA final policies in January 2025.
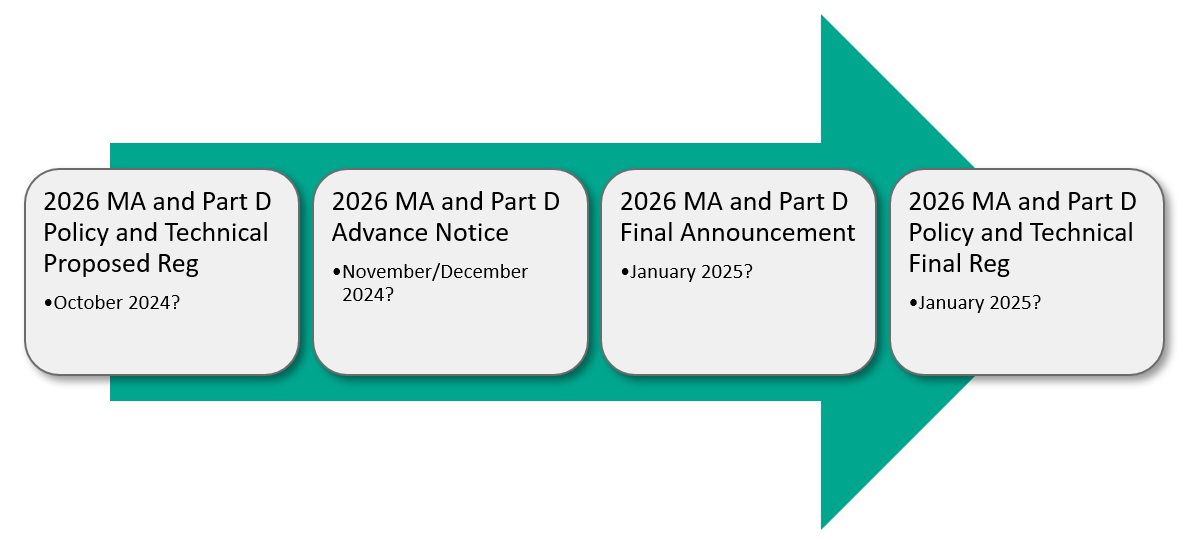
With respect to the MA and Part D programs, the Contract Year 2026 MA and Part D Policy and Technical Proposed Reg is now in OMB clearance and could be released shortly. This annual proposed reg is usually released in November or December, followed by the MA and Part D Advance Notice a few months later (usually in January or February). The MA and Part D Policy and Technical Final Reg and the MA and Part D Final Announcement typically aren’t released until April. However, in this election year, CMS may decide to finalize MA and Part D policies for 2026 in January 2025, prior to the start of the new administration.
Typical Year (Example: 2025 MA and Part D Policies)

Election Year (Example: 2026 MA and Part D Policies)
Expect the Unexpected
We may see some regs pop up between now and the end of the year that we weren’t expecting at all. A few weeks ago, CMS issued a Fiscal Year 2025 Inpatient Prospective Payment System (IPPS) Interim Final Reg. This reg appeared unexpectedly in OMB clearance last month and had the health policy world wondering what it could be. It turned out that CMS was responding to a court decision affecting hospital wage index values under the IPPS. Following an appellate court decision in Bridgeport Hosp. v. Becerra, CMS revised Medicare wage index values for fiscal year 2025.
Just last week, we received another regulatory surprise. We had been expecting the US Drug Enforcement Administration (DEA) to issue long-term policies related to the prescribing of controlled substances via telemedicine. In fact, a reg titled Telemedicine Prescribing of Controlled Substances When the Practitioner and the Patient Have Not Had a Prior In-Person Medical Evaluation has been with OMB since June 13, 2024 (but has not yet been publicly released). However, last week, OMB received a new reg titled Third Temporary Extension of COVID-19 Telemedicine Flexibilities for Prescription of Controlled Medications. As colleagues at McDermott Will & Emery stated in a recent alert, the presence of this new reg at OMB suggests that the DEA is planning an additional extension of COVID-19 flexibilities for telemedicine prescribing of controlled substances, beyond the current expiration date of December 31, 2024. As the reg title implies, this would be the third such extension by the DEA, and we will likely have to wait longer for the agency to establish permanent telemedicine policies.
While these are some of the major healthcare regs that we may see issued by the end of the year, there could be more surprises. So, when it comes to regulations as we approach the election, remember to expect the expected and the unexpected!
Until next week, this is Jeffrey saying, enjoy reading regs with your eggs.
For more information, please contact Jeffrey Davis. To subscribe to Regs & Eggs, please CLICK HERE.

