McDermott+ is pleased to bring you Regs & Eggs, a weekly Regulatory Affairs blog by Jeffrey Davis. Click here to subscribe to future blog posts.
February 27, 2025 – In the health policy world, nothing gets folks like me more “excited” than expecting and then seeing a new healthcare regulation pop up in the Federal Register. While the content of the reg is usually what public stakeholders focus on, the process the federal government underwent to develop the reg may soon be of interest too— since that process could be changing.
As someone who previously worked in an office that helped “clear” regs for release in the federal government, I have some insight into the regulatory process. However, while I can tell you how things have worked previously, I don’t know exactly what the process and protocols will be under the Trump administration – especially now that President Trump has released an executive order inserting the Department of Government Efficiency (DOGE) directly into the regulatory process.
For more on this topic, listen to our Health Policy Breakroom podcast.
Before diving into that executive order, let’s examine what the process at the US Department of Health and Human Services (HHS) has looked like previously. We’ll use a Medicare payment rule that comes from the Centers for Medicare & Medicaid Services (CMS) as an example.
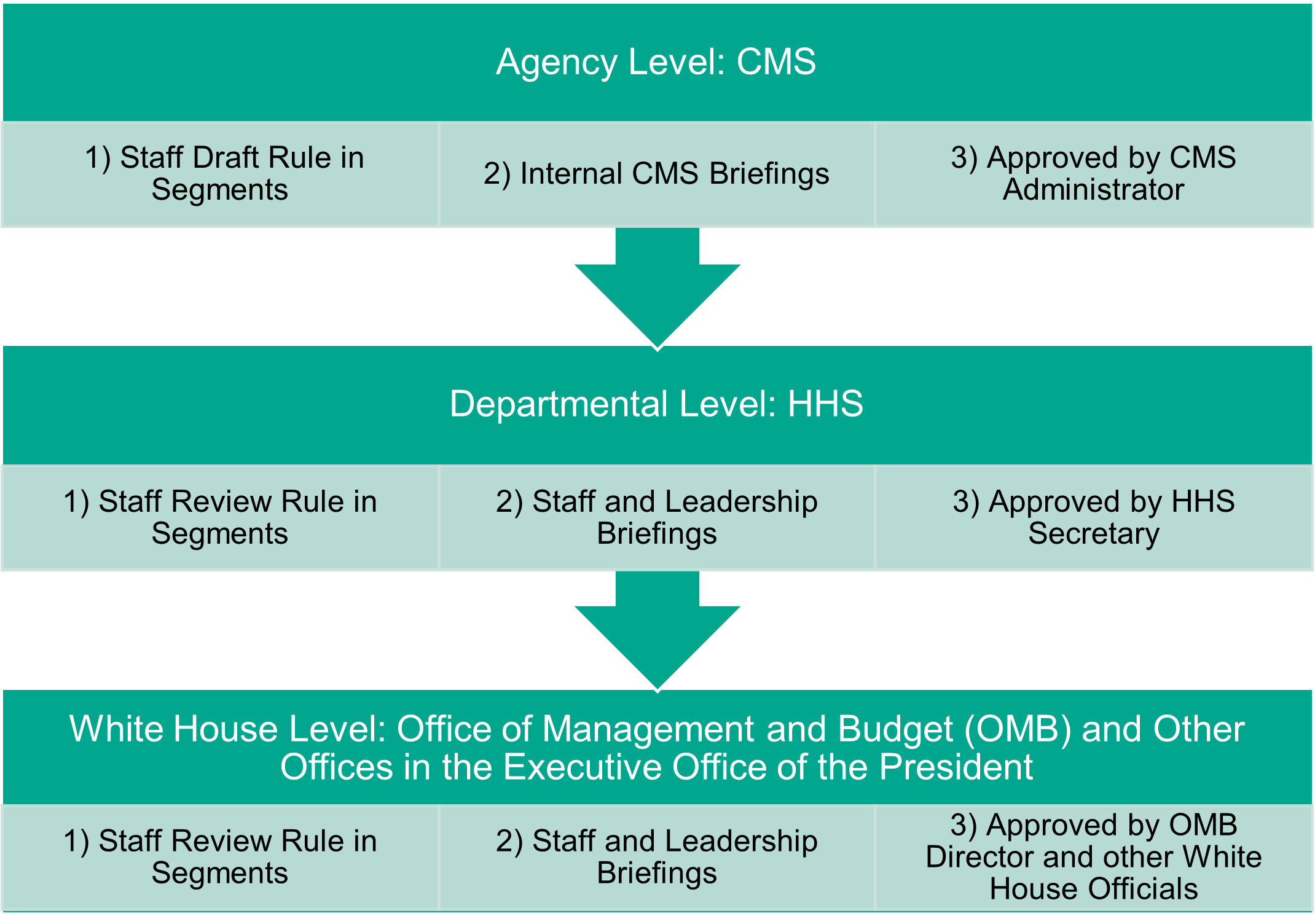
Regs generally move through three levels of clearance: the agency level, the departmental level, and the White House level. Major regs such as the Physician Fee Schedule are so big that they move through the clearance process in segments, allowing policy officials to approve one bite-sized chunk of policies at a time, and then eventually the whole rule before it is released.
At each step of the process, staff and policy officials ensure that the rule aligns with the priorities of the agency, the department, and the White House, respectively. There are many briefings, internal comments and edits, and requests for modifications or deletions of certain policies or sections. And yes, there are plenty of internal policy disagreements (big and small) that must be worked out before a reg is released. I worked in the HHS Budget Office for nearly eight years, and I was heavily involved in the departmental level of clearance. I reviewed Medicare payment rules and policies and briefed HHS policy officials on the financial and policy implications of the rules to ensure that they supported the HHS secretary’s overall objectives.
The public is alerted when rules, or segments of rules, reach the White House level, the last step in the process. At that time, the name of the rule and a high-level explanation of its purpose appears on the OMB Office of Information and Regulatory Affairs (OIRA) Executive Order Submissions Under Review web page. OIRA is the office within OMB that is responsible for handling the White House clearance process (which includes not just OMB, but other offices within the Executive Office of the President, such as the Domestic Policy Council). Once a rule is posted on the OIRA website, the public is allowed to schedule a meeting with OIRA to discuss the rule as long as it remains in the White House stage of the clearance process (if the rule completes this process before the requested meeting is scheduled, the meeting is automatically canceled). Such a meeting is organized by OIRA and usually includes staff and officials at the agency, the department, and the White House. Once the White House clears the rule, the rule is removed from OIRA’s Executive Order Submissions Under Review web page and appears on the Executive Order Submissions with Review Completed in Last 30 Days web page. This effectively means that the rule could be released on the Federal Register at any time.
A rule’s appearance on the Executive Order Submissions Under Review web page does not mean that the rule will be cleared and released in a specific amount of time. Because major Medicare rules move through the clearance process in segments, it is possible that when a rule first appears on the site, the White House has only started to review the first segment, not the whole thing. Major rules usually stay on the OIRA Executive Order Submissions Under Review page for weeks or months before they are removed and eventually issued.
That is how the process generally works. Each administration runs the process a little differently and has certain individuals at each level who get the most involved in the process and in deciding what policies can go forward. There is usually some degree of “learning as you go” as new policy officials go through the process for the first time. As stated in a previous Regs & Eggs blog post, one of the first major healthcare regs the Trump administration must issue is the contract year 2026 Medicare Advantage (MA) and Part D final notice, and perhaps the final MA and Part D reg as well. Since bids from MA plans are due June 2, 2025, the Trump administration will need to act relatively quickly to lock down final policies. After that, the administration will issue proposed regs for Medicare facility payment systems, such as the Inpatient Prospective Payment System. Given these upcoming deadlines, policy officials are beginning the rulemaking process as we speak. In fact, the fiscal year 2026 Inpatient Prospective Payment System proposed rule is on the Executive Order Submissions Under Review web page, which means that the White House has reviewed at least the first segment of the rule.
While the regulatory process is generally expected to be a little messy at the start of a new administration, President Trump’s DOGE regulatory executive order adds an extra consideration. The executive order, dated February 19, 2025, requires that agency heads, in “coordination with their DOGE Team Leads and the Director of the Office of Management and Budget, initiate a process to review all regulations subject to their sole or joint jurisdiction for consistency with law and Administration policy” (emphasis added). Within 60 days of the order, agency heads must identify regs that meet certain criteria, including those deemed to be unlawful or unconstitutional. The executive order also states that the “the Administrator of OIRA shall consult with agency heads to develop a Unified Regulatory Agenda that seeks to rescind or modify these regulations, as appropriate.” The unified agenda is where OIRA lays out all the regulations (and now deregulations) that an administration plans to release. It is revised twice a year. The Trump administration rescinded the Biden administration’s last unified agenda and has yet to issue one of its own. With respect to the regulatory process itself, the executive order states that agencies should continue to submit regulations for review through OIRA, but that agency heads “shall consult with their DOGE Team Leads and the Administrator of OIRA on potential new regulations as soon as practicable” (emphasis added). The OMB director will issue more guidance as needed on how the regulatory process should be structured going forward.
While the executive order states that OIRA will continue to oversee the White House clearance process, some questions come to my mind regarding DOGE’s overarching role in that process:
DOGE’s involvement could impact both the timing and the substance of at least some rules going forward. For example, if DOGE reviews every rule independently, that could extend the timeline for getting rules out the door. In my experience, adding more cooks to the regulatory kitchen can sometimes slow down the entire process, as OIRA must wait for all the cooks to finish up their review before a rule is cleared. But, it may turn out that adding another cook doesn’t impact timing and rules can still move through the process on schedule.
DOGE could also decide only to review certain rules. As stated in a previous Regs & Eggs blog post, there are two overarching types of regs:
If DOGE decides to review all the discretionary rules but lets certain mandatory rules slide through, that process could impact the content of specific rules. Should DOGE set stringent requirements for discretionary rules that it reviews closely, it could become more challenging and time-consuming to get new discretionary rules through the clearance process. Agencies could attempt to add necessary policy “riders” to mandatory rules to ensure that those policies are issued in a timely manner. As a result, mandatory rules could include policies not directly germane to their overall purpose.
It is also important to consider when DOGE might get involved in the clearance process. Since DOGE is part of the Executive Office of the President, one could assume that DOGE will be part of the White House clearance process. However, DOGE also might get involved earlier, since it is already working directly with agencies and their heads and staff on policies and priorities. Another possible scenario is that DOGE gets the final say on regs before they are released. At the end of the clearance process, agency, departmental, and White House staff and officials tend to work together to hammer out any final policy issues and get the rule out the door. At that point, all officials have already seen and engaged on the rule. If DOGE only comes in at the very end of the clearance process, there could potentially be delays as DOGE team members “catch up” and learn about the issues in the rule.
Another way rules could change hinges on the last factor mentioned above: the way that DOGE and OIRA will require agencies to capture and document cost savings from regs. DOGE and OIRA might call on agencies to restructure the “regulatory burden analysis” and “impact analysis” in each rule. These elements show the overall financial impact of the rule and the “burden” on stakeholders for adopting the rule. In a previous executive order, President Trump directed the heads of agencies “to ensure that the total incremental cost of all new regulations, including repealed regulations, being finalized this year, shall be significantly less than zero, as determined by the Director of the Office of Management and Budget (Director), unless otherwise required by law or instructions from the Director.” That same executive order, discussed in a previous Regs & Eggs blog post, also requires that for each new regulation issued, at least 10 prior regulations be identified for elimination. Impact analyses in rules therefore may need to show how the rule contributes to the goal of producing significant regulatory savings, and identify what rules are being eliminated in accordance with the deregulation requirement. Figuring out how the costs or savings associated with a specific rule contribute to an overall savings target, then documenting that result in the rule’s impact analysis, may take additional time and may cause some delays, at least initially, in the release of certain rules.
There are many more questions than answers at this point, but I will continue to keep you updated as we learn more about how the regulatory process will operate under the Trump administration.
Until next week, this is Jeffrey saying, enjoy reading regs with your eggs.
For more information, please contact Jeffrey Davis. To subscribe to Regs & Eggs, please CLICK HERE.